
stability of alkoxides depends on solvationīulky alkoxides are less solvated and less stable (stronger bases).most commonly made by direct reaction with active metals.deprotonation of alcohols gives alkoxide anionsĬH 3OH + NaNH 2 -> NH 3 + CH 3O - Na+ (sodium methoxide).Thiols have lower boiling points and are less soluble in water than alcoholsġ8, 17, 16, 15.5 (compare H 2O: pKa = 15.7).Thiols show little association by hydrogen bonding.H-bonds hold together the strands of DNA ("Velcro" effect).(pentane and diethyl ether both boil at about 35°, but 1-butanol has a bp of 117°) Or alkyl halides (polar, but no H-bonds) alcohols have higher boiling points than alkanes (nonpolar).covalent O-H bond strength ~ 100 kcal/mole.in alcohols, O lone pairs interact with polar H bonds.attraction between the positive end of one dipole (an H bonded to F, O, or N - atoms of high electronegativity) and the negative end of a dipole, usually a lone pair on F, O, or N.The attraction between the positive end of one dipole and the negative end of another They interact with themselves and with other polar compounds by dipole-dipole interactions.Alcohols are polar compounds due to the polar C-O and O-H bonds.The bond angle about sulfur in methanethiol is 100.3°,Įxplained as greater p character in the bonding orbitals.The bond angle about oxygen is about 105°,Įxplained as greater repulsions of the lone pairs.sp3 O with two covalent bonds and two lone pairs.Common names: name the alkyl group bonded to sulfur followed by the word mercaptanĮxample: 2-butanethiol or sec-butyl mercaptan.the parent is the longest chain that contains the -SH group.more than one alcohol named as a -diol, -triol, etc.name other substituents and multiple bonds as usual.the direction of numbering gives it the lower possible number

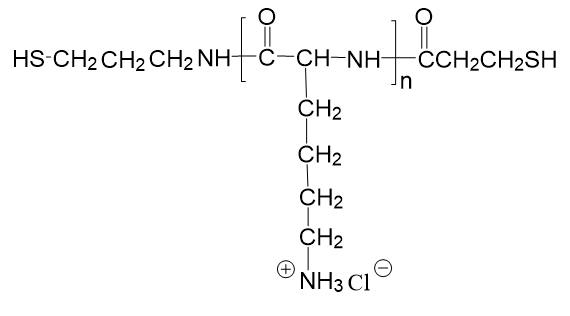
based on the carbon the OH group is attached to:.Oxidation of DTT results a stable six-membered heterocyclic ring with an internal disulfide bond.Dr. Like 1,2-ethanedithiol, propanedithiol forms complexes with metals:įe 3(CO) 12 + C 3H 6(SH) 2 → Fe 2(S 2C 3H 6)(CO) 6 + H 2 + Fe(CO) 5 + COĪ naturally occurring 1,3-dithiol is dihydrolipoic acid.ġ,3-Dithiols oxidize to give 1,2- dithiolanes.Ī common 1,4-dithiol is dithiothreitol (DTT), HSCH 2CH(OH)CH(OH)CH 2SH, sometimes called Cleland's reagent, for to reduce protein disulfide bonds.
The process is foundation of the umpolung phenomenon. When derived from aldehydes, the methyne C-H group is sufficiently acidic that it can be deprotonated and the resulting anion can be C-alkylated. It is employed as a reagent in organic chemistry, since it forms 1,3- dithianes upon treatment with ketones and aldehydes. Propane-1,3-dithiol is the parent member of this series. The dithiol of 1,3-dithiole-2-thione-4,5-dithiolate 2- is also known. The parent aromatic example is benzenedithiol. Examples include dimercaptopropanesulfate (DMPS), dimercaprol ("BAL"), and meso-2,3- dimercaptosuccinic acid.Įnedithiols, with the exception of aromatic examples, are rare. Some dithiols are used in chelation therapy, i.e. (HS) 2C 2H 4 + RCHO → RCHS 2C 2H 4 + H 2O Ethane-1,2-dithiol reacts with aldehydes and ketones to give 1,3-dithiolanes: 1,2-Dithiols Ĭompounds containing thiol groups on adjacent carbon centers are common. Upon heating, gem-dithiols often release hydrogen sulfide, giving the transient thioketone or thial, which typically convert to oligomers. Examples include methanedithiol, ethane-1,1-dithiol, and cyclohexane-1,1-dithiol. Their stability contrasts with the rarity of geminal diols. They are derived from aldehydes and ketones by the action of hydrogen sulfide. Geminal dithiols have the formula RR'C(SH) 2.


 0 kommentar(er)
0 kommentar(er)
